Abstract

Background: The incidence of renal failure in multiple myeloma (MM) is nearly 50% and portends a poor prognosis. Patients with MM and end stage renal disease (ESRD) on dialysis have lower rates of hematopoietic stem cell transplantation (HSCT), presumably from lower referrals due to perceived high transplant-related morbidity and mortality. For those who undergo HSCT, survival outcomes have only been reported scantily. The objective of our study was to determine the HSCT utilization rate and long-term survival benefit among MM patients on dialysis using the US Renal Data System (USRDS), a national database for ESRD patients in the US.
Methods: All ESRD patients in the USRDS database who initiated dialysis between 2004 and 2015 and had a diagnosis of MM were included. Diagnoses and HSCT were identified using international classification of disease (ICD) 9/10 codes in hospital, detailed or physician/supplier claims. Those who were less than 18 years of age, had missing data on age, race, sex, or ethnicity, or had no follow-up due to death at the time of entry into the USRDS were excluded. All statistical analysis was performed using SAS 9.4 and statistical significance was assessed using an alpha level of 0.05. Descriptive statistics were determined overall, by HSCT status, and by mortality. Simple logistic regression models were used to assess the potential for confounding of the demographic and clinical variables with HSCT status. Cox Proportional Hazards models were used to examine the association of HSCT and mortality.
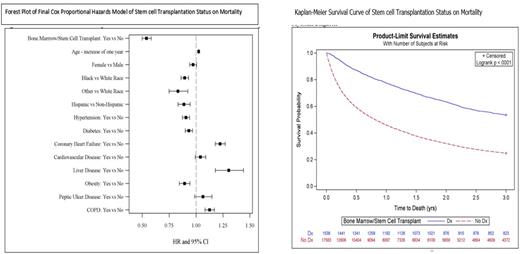
Results: In the study period, there were 19,121 ESRD patients with MM. The average age was 69.8 years (SD=10.8), 43% were female, 28% black and 2.8% other race; 8.5% were Hispanic. A total of 1538 (8%) patients had a HSCT. Kaplan-Meier survival curves showed significantly higher survival for those who received a HSCT versus those who did not receive a HSCT (p=0.0001). The mean time to death among those with HSCT was 2.15 years (SE=0.03) compared to those without a HSCT was 1.29 years (SE=0.01). The median time to death was 0.82 years (95% CI: 0.79-0.85) for those who did not receive a HSCT and was not reached among those who received a HSCT. Those who had a HSCT had a lower risk of death (HR=0.54 95% CI: 0.50-0.58) during the observation period than those who did not, controlling for demographic characteristics and clinical diagnoses. Demographic and clinical diagnoses that were associated with a decreased odds of receiving a HSCT included increasing age, black or other race, hypertension, diabetes, coronary artery disease (CAD), congestive heart failure (CHF), arrhythmias, inflammatory bowel disease (IBD), cerebrovascular disease (CVD), liver disease, obesity, peptic ulcer disease (PUD), and chronic obstructive pulmonary disease (COPD). Of the patients that received a HSCT, factors that led to an increased risk of death included, increasing age, CHF, liver disease, and COPD. Variables that were significant in the final model and were associated with a decreased risk of death included black or other race, Hispanic ethnicity, hypertension, diabetes, and obesity. Obesity is known to have a survival benefit among ESRD patients. In our study, obese patients with MM and ESRD had a lower risk of death post HSCT compared to those who were non-obese.
Conclusions: There is very low utilization of HSCT in patients with MM and ESRD. In our study, those who underwent a HSCT had a lower risk of death compared to those without a HSCT. A strong emphasis on early referral might improve the odds of transplantation and survival advantage in this population. Black race patients with MM and ESRD had lower odds of receiving a HSCT. Further studies accruing diverse patient populations to reduce racial disparities among patients eligible for HSCT should be encouraged.
Disclosures
Kolhe:Perkin Elmer: Honoraria, Research Funding; Agena: Honoraria, Research Funding; Qiagen: Honoraria, Research Funding; Cepheid: Honoraria; PGDx: Honoraria, Research Funding; Illumina: Research Funding; Bioanano INc: Honoraria, Research Funding, Speakers Bureau. Cortes:Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Sun Pharma: Consultancy, Research Funding; Biopath Holdings: Consultancy, Current equity holder in private company; Abbvie: Consultancy, Research Funding; Forma Therapuetic: Consultancy; Gilead: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; Kartos: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.